8. Hazardous substances
- Note GHS pictograms and safety data sheets for hazardous substances!
-
The registry of hazardous substances in the laboratory must be updated upon receipt of new hazardous materials by the person who requested/ordered them!
Hazardous substances pose a hazard in the laboratory. These are substances or mixtures of substances which are classified according to their hazard potential.
The danger of these substances is indicated by hazard symbols, as well as H and P phrases according to the GHS. Hazardous substances can cause acute or chronic health effects in humans.
Pregnant women may only work with toxic and very toxic substances after a risk assessment has been carried out. The hazardous substances include not only chemicals but also drugs, anesthetic gases, etc. Further information on the maternity protection law is available here.
The GHS (Globally Harmonized System) is a global system to classify chemicals, including labels and safety data sheets. The GHS pictograms look visually different in comparison to the old danger symbols. New icons have been added and some old symbols do not exist anymore in the new system. Within the hazard classes, there are also various categories for classification.
GHS pictograms
The GHS pictograms 06, 07 and 08 warn of health risks and are used to indicate toxic properties. The GHS symbol 06 warns about acute toxicity. The toxicity of a substance can be classified into three categories with this symbol (1-3), with category 1 being the highest risk.
The GHS 07 symbol is used to warn about acutely toxic and skin irritating substances. The toxic effect of substances labeled with this symbol classified in category 4 is less severe.
The symbol GHS 08 warns about toxic substances. The toxicity is specific target organ toxicity (STOT). Substances that are labeled with this symbol can also be carcinogenic, mutagenic and toxic to reproduction (CMR).
For all dangerous substances there are safety data sheets available. These data sheets contain information on the composition of hazards and first aid measures, storage and disposal. Location of safety data sheet files: (a) Animal preparation lab: in the cabinet above the sink. (b) RF lab: in the cabinet on the corridor between the two entrance doors.
All hazardous substances are listed in a register of hazardous substances. Upon delivery of a new hazardous substance the person in charge has to ensure that the appropriate safety data sheet (usually included with the delivery of chemicals, www.sigma-aldrich.com) is added to the register.
9. Anesthetics
- Working with isoflurane should always be done under the fume hood and with special attention!
- After inhalation of isoflurane immediately seek fresh air!
- After skin contact: wash with plenty of cold water!
- After eye contact: rinse immediately with plenty of cold water and with the eyelid held wide open. Do so for 15 minutes and consult a doctor!
- In the event of an accident (e.g. accidental spill of isoflurane) the room has to be evacuated! The accident service (Eonova) and the S1 range officer must be notified!
- Access to the contaminated room is only allowed when wearing a breathing mask (located on shelf in the corridor; requires attachment of filter!!)
-
To absorb and bind liquid isoflurane special granules (Absolyt, located on shelf in corridor) should be used.
ISOFLURANE is an anesthetic gas that is used for anesthetizing mice and rats and is therefore another potential source of danger. As possible vapours should not be inhaled, as they may cause drowsiness and dizziness and can impair the ability to respond. Studies have shown that the uptake of Isofluran can increase cancer risk and fertility may be impaired. Furthermore, isoflurane is suspected of causing a premature Alzheimer’s disease. Working with isoflurane should always be done under a fume hood and with SPECIAL CAUTION!

In case of an accident with isoflurane despite warning of hazardous substances you should immediately take first aid action. Immediately after inhalation of isofluran fresh air should be supplied, if necessary, artificial respiration or mechanical ventilation. In case of skin contact you should wash with plenty of cold water. Contaminated clothes should be removed directly. After eye contact rinse immediately with plenty of cold water with the eyelid held wide open for at least 15 minutes and consult a doctor in all cases. People who have shallow isoflurane should get a gastric lavage as soon as possible. An interim use of PEG as a rinsing agent may be beneficial. The intake of active coal is another first aid action. Poeople in question should not vomit.
ISOFLURANE as a hazardous substance
In exceptional situations, such as accidental release of isoflurane, the room should be evacuated immediately. For cleaning up the room you are only allowed to enter this room wearing a full face mask with filter for organic vapors (e.g. mask 3M – 4255 FFA2P2, see S1-floor). The Isofluran can be absorbed with absorbent granules (e.g. Absolyt). Than the granules can be collected and placed in a lockable box. Treatment according to toxic organic material.
In any case, the disaster service EONOVA and the S1 range officer must be contacted.

10. Genetically modified organisms (GMOs) (for laboratory work)
- To protect the animals from infections protective clothes must be worn, consisting of a lab coat, gloves, cap and mask!
- A GMO is an organism whose genetic material has been altered in a way that does not occur under natural conditions. In our lab GMOs are almost exclusively mice.
- GMOs are classified according to their hazard potential in four safety levels (S1 represents the lowest risk).
- Eating and drinking is prohibited in the S1 area!
- For each project using GMOs records must be kept!
- S1-contaminated gloves have to be taken off before using common objects (e.g. phone), and before leaving the S1 area.
- Escaped GMOs (mice) must be captured immediately!
-
Contaminated skin and cut or stab wounds and injuries should be thoroughly disinfected and reported to the project leader! A doctor should be consulted!
Everyone should be the aware that it may possibly come to contact with hazardous substances and animals when entering the S1 area. Access to this area is via S1-lock. Here your shoes have to be cleaned and in case of strong dirt you have to wear overshoes. To protect the animals against infection you also have to wear protective clothes consisting of lab coat, gloves, cap and mask.


The productions of genetically modified organisms (GMO) or usage, storage, destruction or disposal of GMO are genetic engineering operations. A genetically modified organism (GMO) is an organism whose genetic material has been altered in a way that does not occur under natural conditions. Genetic engineering facilities are facilities in which genetic engineering works are carried out in a closed system and where barriers exist to limit the contact of the organisms to people and the environment.
Genetic engineering operations are classified in four levels of security (§7 GenTSV) according to their hazard potential:

At security level 1 you must not eat or drink in these rooms. Pipetting by mouth is prohibited. S1 genetic engineering operations must be carried out only in approved laboratories of level 1. Before the start of each new project and during the study records must be kept (“experiment Records” database). When working with genetically modified organisms you should not produce aerosols. Work surfaces must be disinfected after completion of the work. S1-contaminated gloves have to be taken off before using common objects (e.g. phone), and before leaving the S1 area. The windows and doors of the S1 area must be kept closed during the work.
How to behave in case of danger in S1 area
Escaped GMOs must be captured immediately! All contaminated items and surfaces should be disinfected with 2% Kohrsolin (let sink in for at least one hour). In case of spilling contaminated material you have to disinfect the affected area immediately and give the material into a container for autoclaving. Contaminated skin and cut or stab wounds and injuries should be thoroughly disinfected and report to the project leader and a doctor (Durchgangs-Arzt, HELIOS-Klinikum Berlin-Buch, Schwanebecker Chaussee 50, 13125 Berlin) should be consulted.
11. Waste management (for laboratory work)
- Hazardous substances must never reach the sink, they must be disposed separately!
- Chemical solid and liquid wastes have to be separated!
- Materials contaminated with animal waste are collected and disposed in B waste!
- Needles must be disposed of in special boxes!
-
S1-waste must be rendered harmless by autoclaving!
S1 waste is separated from household waste and recyclable waste from kitchen and office. The solid wastes are classified according to Green Dot (yellow bag), B waste (black bag) and chemical solids (white barrel). Packaging labeled with a green dot, Styrofoam and foil are collected in the Green Point container. Gloves, syringes, rags, bedding, blood, feces, and all things that are contaminated with animal material are collected in B-waste. With the white ton for chemical solid wastes are all solid materials that are contaminated with chemicals disposed of.
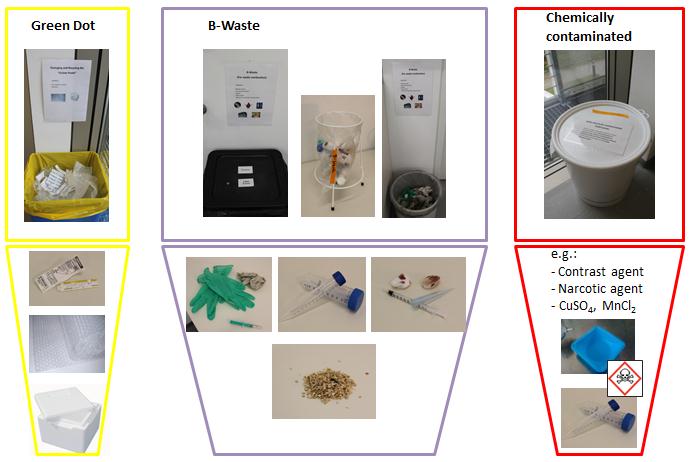
Liquid waste in S1 and HF lab are separated into waste containers for staining solution and solvent. When disposing it always comes with on the concentration of liquids! For example, manganese chloride solution with a concentration of less than 5% is disposed of in a canister for staining solutions, with a concentration of 5%, the solution must be disposed of as heavy metal waste upwards. Hazardous substances may never reach the sink; they must be disposed of separately!

Needles must be separated in special boxes (left). Here, too, a distinction is made between the B waste and chemically contaminated waste. Liquid and solid S1 waste and contaminated wastewater have to be collected safely and rendered harmless by autoclaving (right).


12. General Safety
- Observe warning signs! Better safe than sorry!
-
First-aid kits are located on each floor!
In the laboratory you should always work to the best of your knowledge and belief, therefore, applies in principle: it is better to be safe than sorry!
The most important warnings:
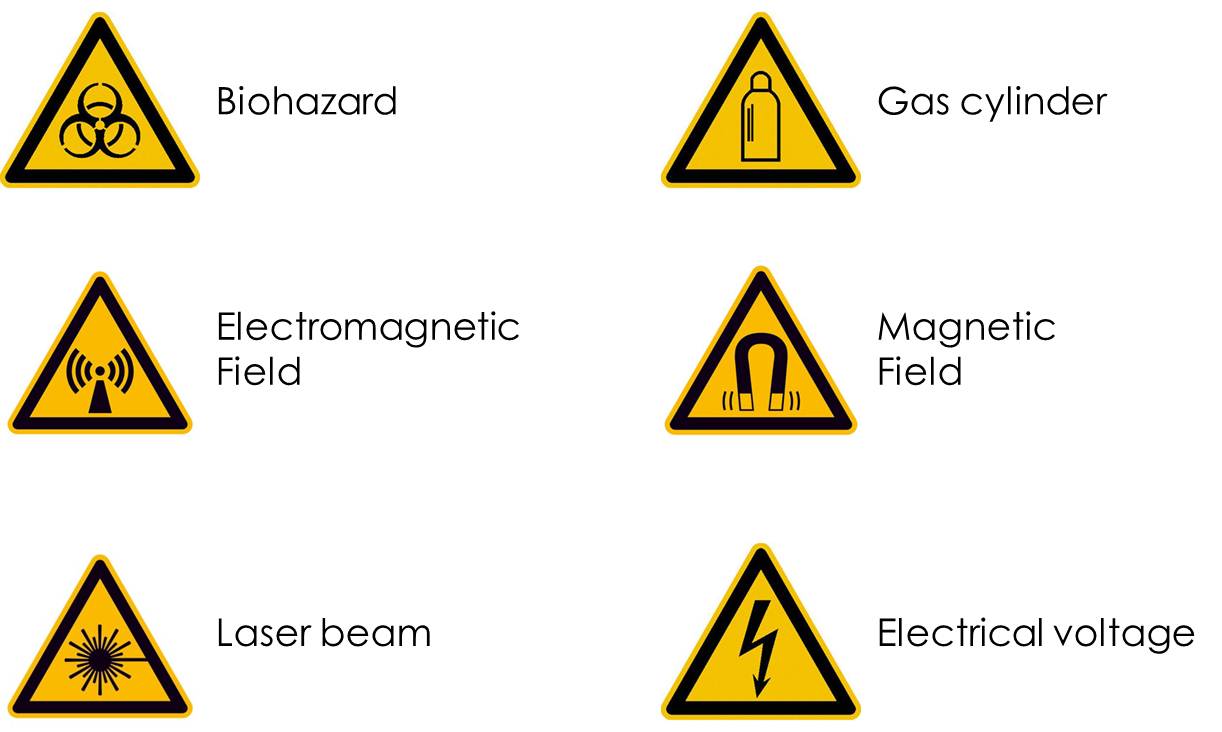
The main prohibition- and mandatory-signs:
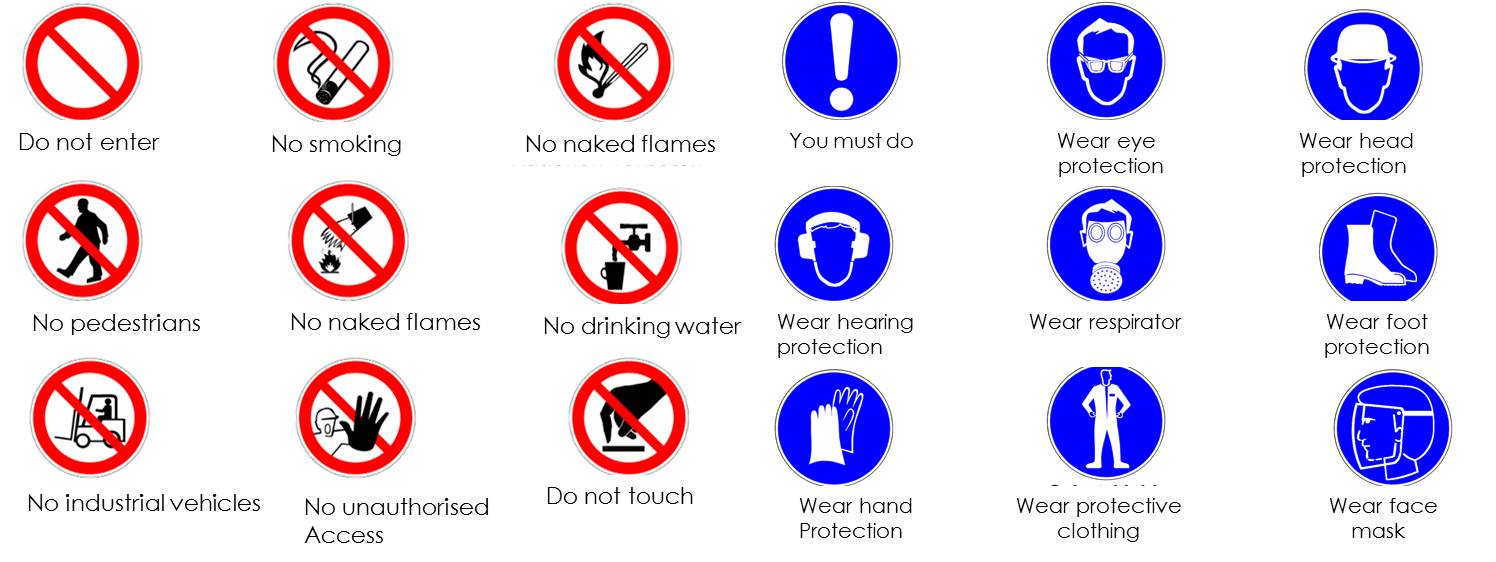
The emergency equipment, such as electrical emergency, the magnet quench, the safety shower, eyes shower, and the First Aid Kits are marked as such. The First Aid Kits are located on each floor. On the first floor in the laboratory there is a full body shower and eye bath. Another eye bath is located in the preparation room on the ground floor.

For a full view of steps for First Aid, click on these poster: 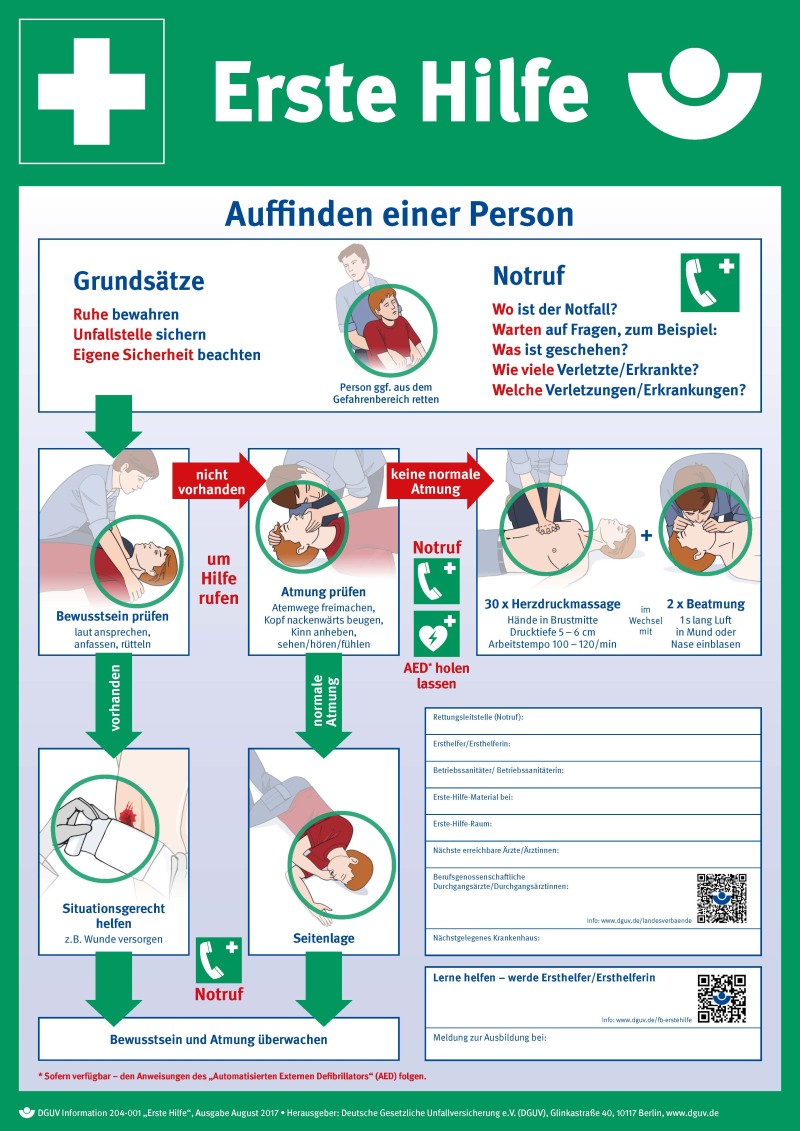
13. Fire protection
- Emergency number: (0)112 or 3333
- In the MR room may ONLY be used MR-safe fire extinguisher! Note: There are building both magnetic and non-magnetic fire extinguisher!
- Readily combustible waste must be disposed of shortly!
- Flammable liquids or gases shall be stored in the safety cabinet!
- Emergency exits and escape routes should always be known!
-
Director of operations of the fire department must be made aware of the dangers of the MRT!
In case of fire it is always important to ensure that only non-magnetic fire extinguishers are used in the scanner area. Signs bearing the inscriptions “forbidden to use in the magnet room” and “use in the magnet room” give appropriate instructions. ATTENTION: in B.U.F.F: both magnetic and non-magnetic fire extinguishers are distributed!

Fire and accident prevention
Use of open flames, and smoking in the building is strictly prohibited. Welding and brazing operations must be carried out only with a written approval of the conductor. Readily combustible wastes are always ready to dispose of and combustible liquids or gases (from 1L container size) must in safety cabinets (in S1 laboratory) are stored. All electrical appliances must be switched off after use.
When entering an unknown building should always inform about the escape and rescue routes. These are always to be found in the escape and rescue plan and are marked by green emergency signs in the building. Corridors, stairs and exits must be kept clear. Emergency exit doors must be closed, but may not be completed.
Knowingly misuse of personal protective equipment (eg fire doors) can be prosecuted by imprisonment or a fine.

Detection and extinguishing equipment
In an emergency, the fire protection systems are to be operated and the emergency number (0)112 or 3333 to dial. Fire extinguishers are located in the corridors and scanner rooms. On each floor fire blankets are provided, which can be used for small fires or for personal protection. For your own safety, you can prepare yourselves by taking part in fire drill by the AG Sicherheit for the worst.

How to behave in case of fire
- Keep calm
- Respond rapidly, but not frantically
- Dail (0)112 or 3333
information: - What happened?
- Where is it happening (Bldg., room no.)
- Name of Reporter
- Press emergency stop
- If possible, close window in the fire room
- All employees are required to leave the building (staging area = rear exit)
- Do not use elevators in case of fire
- All employees have to participate in the rescue
- Upon arrival of the fire brigade its team leader is to point out the dangers of MRI, his instructions are to be followed
Important Contacts in B.U.F.F. are here to find again.
14. Clinical Studies
- Clinical studies must be approved by the ethics committee!
- Each volunteer is informed by an investigator and asked about possible contraindications.
- Each volunteer must sign a consent form.
-
The communication between volunteers and researchers investigator is always running on the study assistance.
What is a clinical study on B.U.F.F.?
A clinical study involves volunteers. Initiation takes place in most cases by an investigator. In a study the feasibility of new technologies is demonstrated. Each study requires approval by the ethic committee.
Exemplary Approval overview
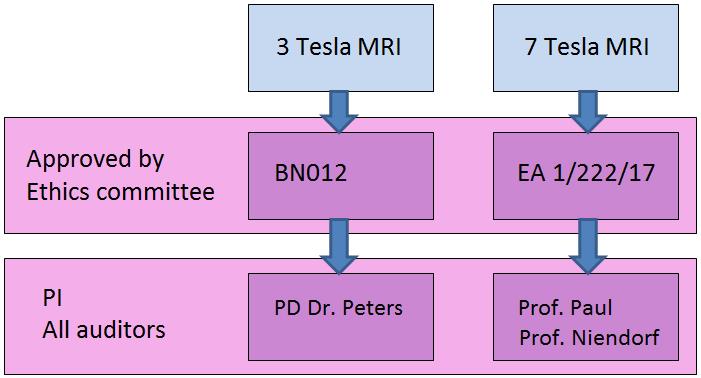
Role of the study assistance
The study assistance communicates with the Principal Investigator (PI) and the researchers and prepares all the needed study documents. They recruited patients and controls and checks them for MRI compatibility. The communication between the volunteer and the investigator (Dr. Mehling) also runs over the study assistance. Furthermore, she reserves the slots on the device and prepares the volunteers on the MRI before. Each volunteer who participates in a study, a consent form must be signed by an informed consent discussion. Only after complete documentation (More information) a volunteer may be put into the MRI.

Required information three weeks prior to study entry
Before the study is a brief summary of the objectives, a study plan or protocol and a schedule must exist. There must be information about possible doses of contrast agents.
SOP summary
- Make sure that you adhere to the work instruction
- Make sure that you include the study assistance
- Each volunteer is to introduce the investigator
- Each volunteer requires its own patient clothing
- Food and drinks are not allowed in the scanner area
- After completion of work, the scanner area is restore to its original state so that the next user finds a clean workplace
- It is imperative that the PI/researcher collects its data and secures
- Failure to follow these instructions will result in the loss of the scan permission!
Correct behavior for dealing with volunteers during normal operation:
- Volunteer must wear patient clothing
- Volunteer instruction
- Control of all parties before they enter the scanner room (volunteers, doctors, MTAs, nursing stuff, partners, guests, etc.)
- All metal parts have to be stored, otherwise wearing of metal parts can lead to combustion!
- Hearing protection for those who remain in the room during the scanning
- Emergency bell for the volunteer
- No companion/caregivers in the scanner room
- Close the door!
Volunteer instruction and contraindications
Volunteers are informed prior to any investigation about the risks of MRI and asked about contraindications. People with implants, metal fragments, body piercings or other metal parts in the body, as well as pregnant women and people with tattoos are not allowed in MRI machine for security reasons.
People with claustrophobia, ICU volunteers, children and people with insufficient thermoregulation represent a risk group and may be examined only after examination of the investigator.